News & Stories
Read the latest news and stories in the SickKids newsroom. Looking to interview someone? Connect with our media team.
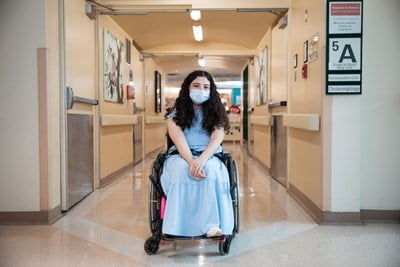
April 24, 2024
Melika Ghanaati on growing up at SickKids and her journey for answers
Long-time patient Melika Ghanaati shares her ongoing journey to diagnosis, and what SickKids has meant to her.
April 18, 2024
Inherited gene variations reveal precision therapy potential for rare cancer syndrome
Global research led by SickKids finds new strategies to help diagnose and treat a rare and aggressive cancer predisposition syndrome.

April 17, 2024
Designed with input from patients, families, staff and the community, this strategy will be a blueprint for a more human-centred experience across care, research and education at SickKids.

April 8, 2024
Dr. Abhaya Kulkarni appointed Surgeon-in-Chief and Chief of Peri-Operative Services
Through 20 tremendous years at SickKids, Kulkarni has proved himself multiple times over to be a gifted and trusted physician, researcher, educator and leader.

March 29, 2024
Genetic causes of cerebral palsy uncovered through whole-genome sequencing
Canadian scientists demonstrate that cerebral palsy may be caused by both genetic and environmental factors, informing diagnosis and care for the diverse condition.
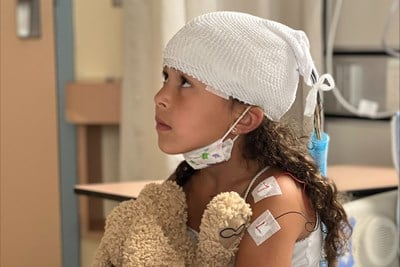
March 26, 2024
From symptoms to diagnosis: Eden's epilepsy story
When Eden was four, she was diagnosed with epilepsy at SickKids. For Purple Day, observed annually on March 26, Eden’s mom Andrea shares their experience of receiving an epilepsy diagnosis.
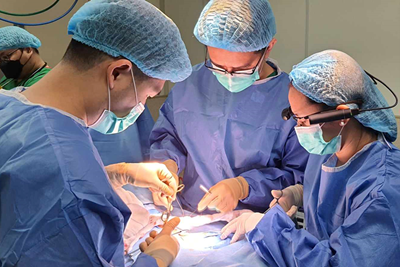
March 25, 2024
SickKids surgeons use “smart glasses” to telementor in low-resource countries
Mentors can share specialized surgical skills in real time to mentees across the globe using a virtual interactive presence.

March 14, 2024
SickKids program provides integrated, trauma-informed care for pregnant and parenting adolescents
The program helps address recent findings that teen pregnancy in Ontario is associated with a significantly increased risk of premature mortality for adolescent mothers.

March 13, 2024
SickKids-led projects receive $17.9 million from the Canada Foundation for Innovation
The funding will support SickKids research into understanding memory formation, increasing genomics capacity in Canada and advancing precision child health.

March 8, 2024
International Women's Day: Dr. Jennifer Quon, SickKids' first female paediatric neurosurgeon
In her own words, Dr. Quon reflects on experiences, challenges and supports that helped shape her career.

February 29, 2024
SickKids joins national initiative for data sharing to help diagnose rare disease
SickKids announces its collaboration with CHEO and other Canadian Institutions as part of the All for One Precision Health Partnership.

February 29, 2024
Overcoming barriers to conducting clinical trials in childhood rare disease research
SickKids researchers use novel approach to show that a medication reduces the rate of liver disease progression in children with Alagille syndrome.

