Diagnostic & Interventional Radiology
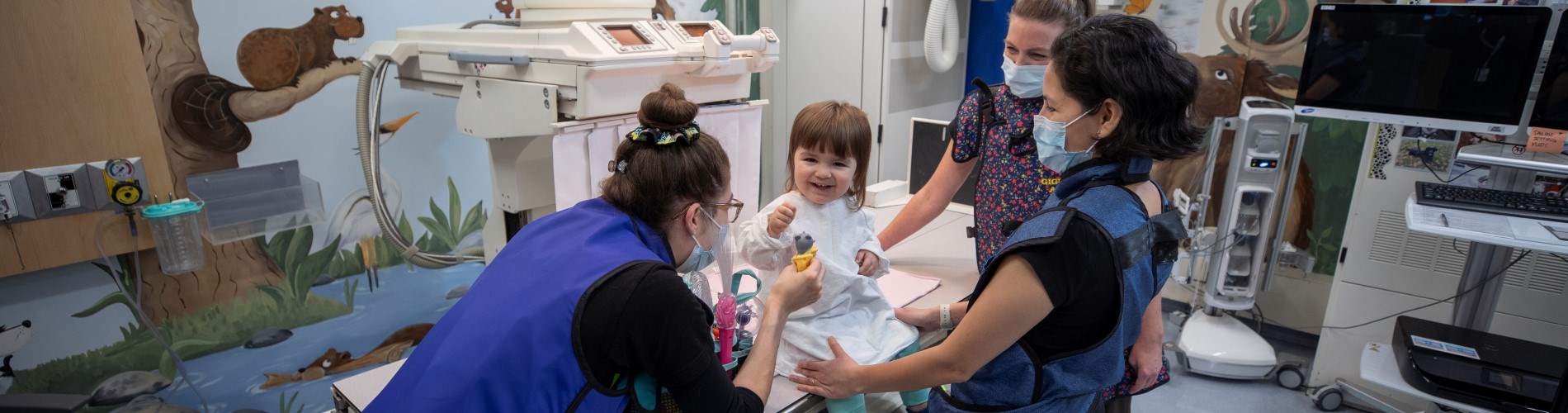
The Department of Diagnostic & Interventional Radiology (DIR) is dedicated to improving and enhancing patient care and the health of children. Our mission is to be a benchmark paediatric radiology site by embracing new technologies and utilizing state-of-the art equipment efficiently and effectively.
Our department is subdivided into specialties: CT, GI/GU Fluoroscopy, Image-Guided Therapy (IGT), MEG, MRI, G Tube Feeding Program, Nuclear Medicine, Ultrasound and X-Ray. Collaborating with the radiologists and technologists in these subdivisions as well as the dedicated staff who run Information Services, Research, Nursing and Quality and Safety contribute to making every patient's visit in DIR run smoothly. We provide an environment that supports and promotes clinical, academic and research excellence, and builds departmental clinical research expertise focused on the strengths of the adjacent clinical and research community.
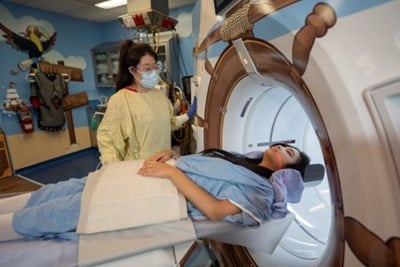
Learn more about CT scans and how to prepare for your appointment.
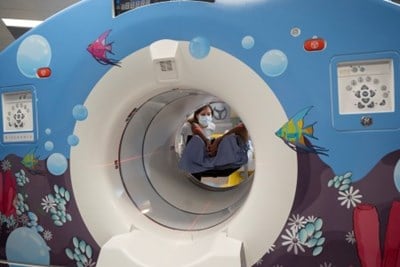
PET (Positron Emission Tomography)
Learn how to prepare for your appointment and more about PET scans.
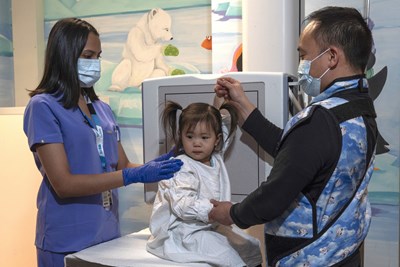
Learn more about X-ray (General Radiography) scans and how to prepare for your appointment.
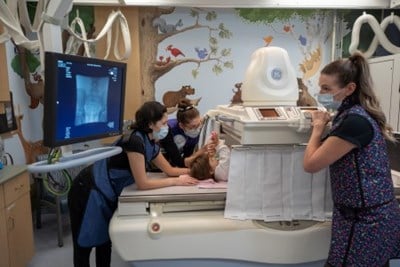
GI/GU (Gastro-intestinal/Genito-urinary) Fluoroscopy
Learn more about GI/GU Fluoroscopy scan and how you can prepare for this procedure.
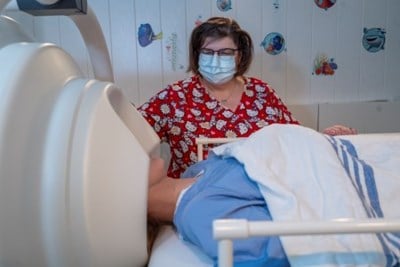
Learn how we study the brain using MEG scans and how you can prepare for your appointment.

MRI (Magnetic Resonance Imaging)
Learn how to prepare for your MRI scan and what this non-invasive procedure entails.
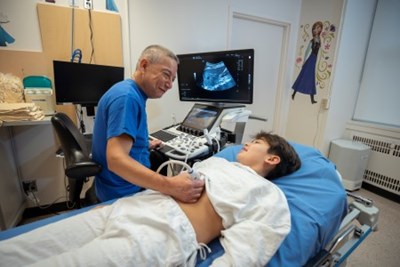
Learn more about the ultrasound scans and how to prepare for this non-invasive procedure.
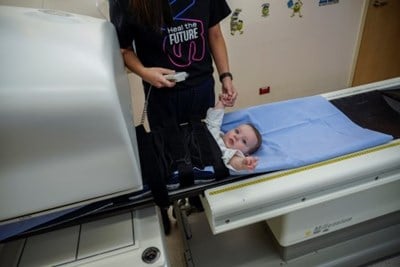
Learn more on what to expect and how to prepare for your appointment with Nuclear Medicine.
Learn more on what to expect and how to prepare for your IGT appointment.
Learn more on what to expect and how to prepare for your appointment with G Tube.
Meet the team

Birgit Ertl-Wagner
Dr. Ertl-Wagner is the Chief of the Division of Neuroradiology and Associate Chief of Diagnostic and Interventional Radiology at SickKids, as well a Senior Associate Scientist in the Neurosicences & Mental Health program at SickKids Research Insitute. She has been the Derek Harwood-Nash Chair in Medical Imaging since 2018. Her research is highly interdisciplinary and collaborative, with a focus on translation between basic science and clinical research. She has worked with a broad spectrum of methodological approaches, including phase contrast imaging, functional MR imaging, diffusion tensor imaging, MR spectroscopy and various perfusion techniques.

Christine Hill
Christine Hill joined SickKids in January 2023 as the Clinical Director for Diagnostic & Interventional Radiology. She is an innovative health-care leader with over 12 years of medical radiation technology leadership experience in complex, academic hospital environments. She is passionate about building health-care systems that drive improvement in patient & family centred care, quality & safety and health-care equity.
- Laura Belsito – Senior Manager, Operations, and G Tube Feeding Program
- Michelle Cote – Senior Clinical Manager, CT/GIGU/IGT/X-ray
- Nicole Bennett – Senior Clinical Manager, MRI, Nuclear Medicine, Ultrasound
- Fatima Lima-Simao – Manager, PACS/RIS, Patient Coordinators and Clerks
- Darlene Murray - Interprofessional Education Specialist
- Mandy Kohli - Interprofessional Education Specialist
- Yuna Dai - Clinical Instructor: Radiography
- Sandra Taves - Clinical Instructor: Ultrasound
- Laura Ripley - Clinical Instructor: MRI
- Sabina Volodina – IGT
- Nancy Ribeiro – Nuclear Medicine
- Jennifer Whitmore – Ultrasound
- Sandi Sutton – Atrium X-ray, GI/GU X-ray and CT
- David Shen – MRI
- Stephanie Holowka – MEG, MRI Safety Officer, Computer Navigation, and 3D Imaging
Radiologists
- Dr. Julien Aguet
- Dr. Joao Amaral
- Dr. Alejandra Bedoya
- Dr. Helen Branson
- Dr. Govind Chavhan
- Dr. Ailish Coblentz
- Dr. Alan Daneman
- Dr. Aurelie D'Hondt
- Dr. Maria Dien Esquivel
- Dr. Andrea Doria
- Dr. Birgit Ertl-Wagner Radiologist-in-Chief
- Dr. Ricardo Faingold
- Dr. Alessandro Gasparetto
- Dr. Samantha Gerrie
- Dr. Mary-Louise Greer
- Dr. George Koshy Chiramel
- Dr. Pradeep Krishnan
- Dr. Christopher Lam
- Dr. Suzanne Laughlin
- Dr. Cathy MacDonald
- Dr. David Manson
- Dr. Claudia Martinez-Rios
- Dr. Elka Miller
- Dr. Prakash Muthusami
- Dr. Oscar Navarro
- Dr. Kamaldine Oudjhane
- Dr. Vivek Pai
- Dr. Dimitri Parra
- Dr. Carmen Parra Farinas
- Dr. Risto Filippi
- Dr. Mike Seed
- Dr. Amer Shammas
- Dr. Manohar Shroff
- Dr. Jennifer Stimec
- Dr. Jeffrey Traubici
- Dr. Reza Vali
- Dr. Shi-Joon Yoo
Scientists (affiliated with the SickKids Research Institute)
WAGS (Wonderful Alternatives to General Anesthesia and Sedation)
Learn more about our wonderful alternatives to general anesthesia and sedation.
Contact & location
- Emergency and Urgent inpatient services: 24 hours a day, seven days a week.
- Map of the DIR department (PDF)
- Visit our programs and services for contact information specific to one of our services.
Requisitions
The Department of Diagnostic and Interventional Radiology (DIR) aims to achieve the highest quality of care for all of our patients. We are committed to providing exceptional services that recognize and respect the complexity of paediatric conditions seen at SickKids, and diversity of our patient population.
We take a systematic approach to continually improve quality of care, enable equitable and timely access to services, further enhance patient safety, introduce advanced technology and equipment, create value for patients and their families, and promote clinical, academic and research excellence. We continually strive towards our mission, to be a global leading the provision of exceptional imaging care for children.
Contact
Senior Manager, Operations: Laura Belsito
Quality Leader: Joey Cheng-Singleton
Medical Physicist: Nicholas Shkumat
MR Safety Officer: Stephanie Holowka
DI Electronic Imaging Department (Film Library)
To get results, or for information about DIR image upload/imports, DIR reports and Pockethealth, please contact us at 416-813-7511, Monday to Friday, from 8 a.m. to 5 p.m.
PACS Support: Monday to Friday between 7 a.m. to 5:30 p.m.
Phone: 416-813-8358
PACS urgent requests: 416-713-0187
Manager: Fatima Lima Simao
Telephone: 416-813-4965
Nursing in DI requires a set of advanced skills and as nurses working within a high tech environment, there is the opportunity to promote and to provide quality-nursing care to children.
The nurses utilize physical assessment skills, perform guided risk assessments, and determine a sedation plan taking into consideration all potential risks to the patient. The care provided by our nursing team spans from initial assessment, to induction, monitoring and recovery.
Interprofessional Education Specialist (RN): Darlene Murray
Clinical Nursing Coordinator, IGT: Wendy Padilla
Clinical Nursing Coordinator, MRI/CT: Annette Martens
Child Life specialists are available from Monday to Friday between 8:30 a.m. and 4:30 p.m. to help prepare and support your child during scans. They can explain the process to your child and help them adjust to the hospital experience.
Contact Child Life Specialists: dir.childlife@sickkids.ca

