Global experts release first international consensus guidelines for a precision genetic medicine
Summary:
The guidelines aim to improve screening and broaden access to antisense oligonucleotide (ASO) therapy.
Researchers at The Hospital for Sick Children (SickKids), working as part of a global collaborative, have released the first international consensus guidelines to screen genetic conditions for their suitability for a precision genetic medicine. The paper was published this month in the American Journal of Human Genetics and includes resources for care providers to apply the guidelines.
Although their use is increasing, genetic medicines customized to a specific rare genetic condition or DNA difference are costly and complex to make widely available. Uncertainty around what genetic diagnoses might be suitable to genetic medicines can cause potentially life-altering delays for patients and frustration for clinicians and researchers working with families.
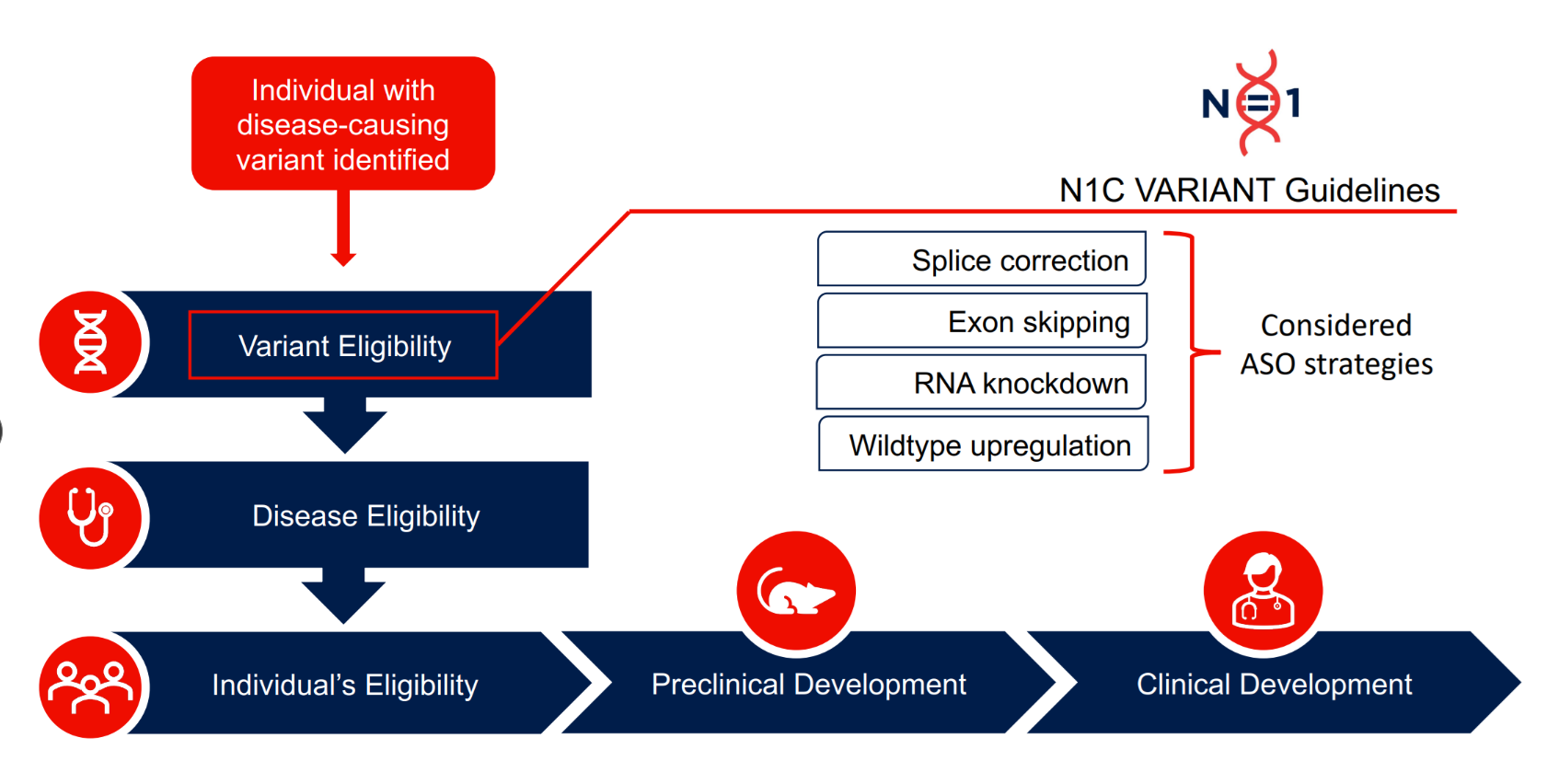
To address this challenge, an international network of experts called the N=1 Collaborative came together to develop guidelines for assessing DNA variants that may be eligible for an antisense oligonucleotide (ASO) therapy, a type of precision genetic medicine.
The recommendations aim to support early detection of potentially eligible diagnoses and expand future access to ASO treatments. The landmark paper also provides a framework that may generalize to other individualized genetic medicines on the horizon, like genome editing.
“This is an important step towards scaling up individualized genetic medicines for the rare disorder community. These guidelines will help us assess each person’s rare genetic diagnosis to understand who could benefit from an ASO and help ensure that families have equitable access to next steps in the therapy development process,” says Dr. Gregory Costain, Co-Lead of Advanced Therapeutics for Precision Child Health, Staff Physician and Scientist in the Genetics & Genome Biology program.
Assessing eligibility for ASO therapy
There are around 7,000 known rare diseases worldwide. Most of these conditions are genetic in origin and while advances in genetic technologies mean up to 50 per cent of cases are diagnosed, targeted therapies are often not available to treat the conditions.
For most children with a genetic condition, their DNA has one or more rare variations. For example, spinal muscular atrophy is caused by the under expression of the gene SMN1, resulting in decreased muscle tone and muscle weakness.
ASOs are short, synthetic strands of DNA or RNA that bind to messenger RNA molecules to regulate the expression of genes. Unlike other genetic therapies that cannot always target the exact strand of DNA involved in a condition, ASOs can target very small areas of a gene with high precision.
“Because patient eligibility for a bespoke therapy like an ASO is multifaceted, defining the molecular genetic criteria that can be used to assess which DNA variants are suitable candidates for this type of therapy is critical,” says Dr. Marlen Lauffer, lead author from Leiden University Medical Center. “By offering accessible resources and examples, we hope to support others in confidently integrating these guidelines into their own practice.”
The future of genetic medicine
Genetic medicines form a core component of Advanced Therapeutics, one of the pillars of Precision Child Health at SickKids that seeks to support rapid development, equitable access, and safe implementation of individualized therapies.
The team hopes that in the future these kinds of guidelines are integrated into clinical practice. Costain notes that with the current pace of advancements in genetic therapy technologies, there is also a need to invest now in clinical trials, implementation research, and policy development.
“ASOs have the potential to radically change how we treat rare genetic conditions,” says David Cheerie, first author on the paper and Clinical Research Project Manager in the Costain Lab. “We want to promote knowledge sharing and enable the broader genetics community to utilize their skillsets in assessing and identifying potentially treatable variants.”
At SickKids, a new Genetic Medicines Program in support of Precision Child Health is being developed to address these challenges and advance precision genetic technologies, while research using these guidelines is already underway to screen patient eligibility for ASO therapy.
This research was supported by a SickKids Restracomp Master’s Scholarship, SickKids Innovators Fund, SickKids Precision Child Health Partnership, Canadian Institutes of Health Research (CIHR), Alexander von Humboldt Foundation, Hertie-Network of Excellence in Clinical Neuroscience, Deutsche Forschungsgemeinschaft, UK Platform for Nucleic Acid, European Rare Disease Research Alliance Therapies (UpNAT), ZonMW, and the Health Data Research UK (HDR UK).

