SickKids scientists discover how brain fluid movement drives cancer spread and ways to fight back
Summary:
Study uncovers new insights into medulloblastoma and the role of fluid shear stress in shaping cancer behaviour.
New research has uncovered how cerebrospinal fluid dynamics in the brain play an important role in driving the spread of medulloblastoma, the most common malignant brain tumour in children. Published in Nature Biomedical Engineering, the study identifies a way to target this fluid-driven process to inhibit tumour spread, known as metastasis. The findings shed light on how physical signals in the brain can influence cancer progression and help guide the development of a new treatment strategy.
As cerebrospinal fluid flows naturally throughout the central nervous system, its movement applies a force parallel to the cells’ surface, known as fluid shear stress.
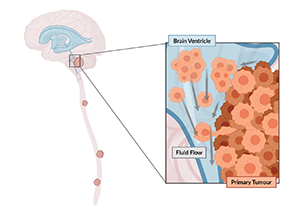
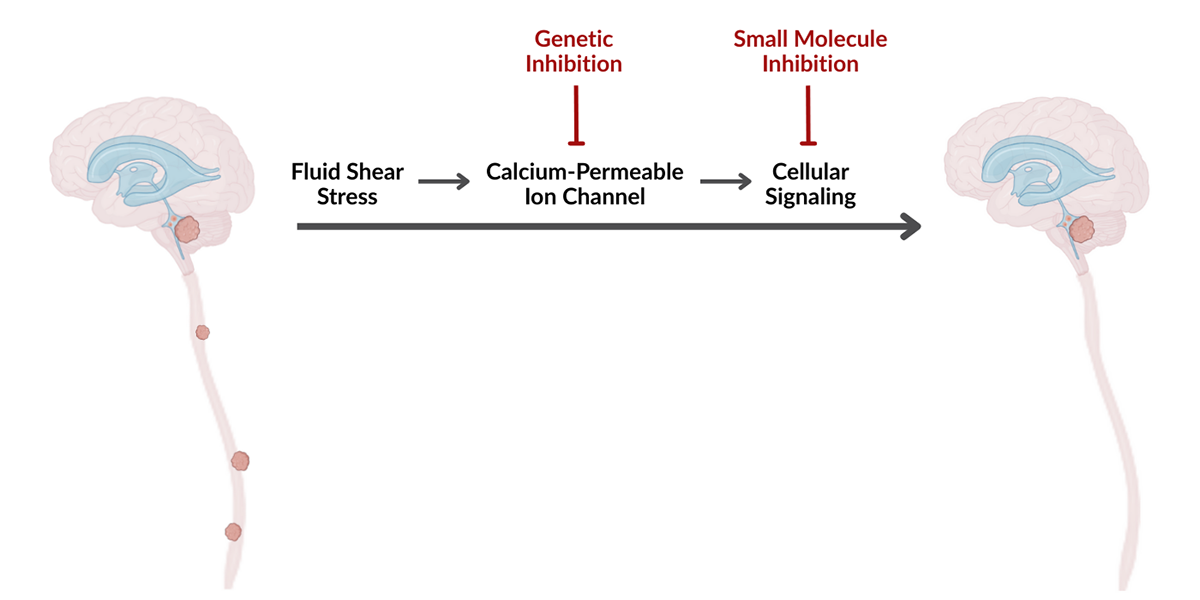
A research team at The Hospital for Sick Children (SickKids) showed that cancer cells detect this mechanical stimulus through a calcium-permeable ion channel on their surface. When triggered, the channel initiates a signaling pathway, enhancing the cells’ ability to leave the primary tumour, enter the cerebrospinal fluid, and survive as they spread across the brain and spinal cord.
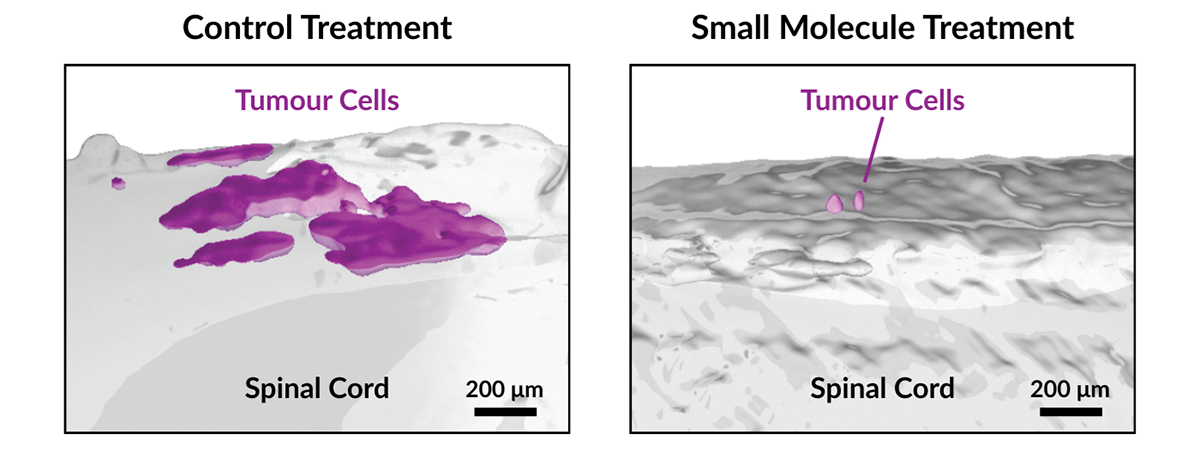
Building on this discovery, the researchers identified two methods to disrupt the signaling cascade. In pre-clinical models, both approaches significantly reduced metastatic spread, offering a promising therapeutic approach for medulloblastoma.
“This study, led by my former PhD student Dr. Hyun-Kee Min, has great scientific and translational significance. Not only have we identified how tumour growth changes fluid flow in the brain and how the altered fluid motion impacts tumour cell behaviour, we also identified a small molecule that can target this fluidic force-dependent cascade with therapeutic potency,” says Dr. Xi Huang, Senior Scientist in the Developmental, Stem Cell & Cancer Biology program and Principal Investigator at the Arthur and Sonia Labatt Brain Tumour Research Centre at SickKids.
Advancing understanding of tumour biology using a multi-model approach
To investigate how fluid shear stress drives tumour spread, Dr. Huang collaborated with SickKids researchers Drs. Brian Ciruna and Madeline Hayes, who contributed expertise in zebrafish modeling and high-resolution imaging.
The team used a multi-model strategy to better understand how fluid shear stress regulates cancer cells across species. This integrative approach provided valuable insight into tumour biology and reinforced the potential of fluid shear stress as a targetable mechanism.
Moving discoveries toward impact
Metastasis is a major clinical challenge in medulloblastoma, with few treatment options available to halt or slow tumour spread.
In collaboration with the SickKids Industry Partnerships & Commercialization (IP&C) office — which supports researchers in advancing discoveries toward clinical application and maintains a technology portfolio showcasing current innovations from SickKids scientists — Huang’s team is now working to translate the findings into safe and effective treatments for patients affected by this aggressive cancer. Huang’s work on medulloblastoma has previously been supported by SickKids IP&C’s Proof of Principle (PoP) Grant Competition, which helps advance early-stage discoveries toward translational impact.
This study was funded by the Arthur and Sonia Labatt Brain Tumour Research Centre, Garron Family Cancer Centre, Ontario Early Researcher Award, Meagan Bebenek Foundation, Brain Tumour Foundation of Canada, Canadian Institutes of Health Research (CIHR) and SickKids Foundation.

