Scientists map proteins key to how malaria spreads in drive for precision vaccines
Summary:
For the first time, scientists have mapped the structure of a malaria protein complex that is key to disease transmission as recognized by blocking antibodies, unlocking new possibilities for designing more precise vaccines.
A new study co-led by researchers at The Hospital for Sick Children (SickKids) and Radboud University Medical Center (Radboudumc) in The Netherlands has revealed the molecular structure of a protein complex critical to malaria transmission. Published in Immunity, the findings offer a foundation for designing more precise and effective vaccines that could help stop the spread of malaria.
Malaria, caused by the Plasmodium falciparum parasite and transmitted through the bite of an infected mosquito, remains a leading global health challenge. While current vaccines can reduce infection rates to some extent, they do not prevent transmission and are not always accessible to children in specific regions. As such, malaria continues to disproportionately affect children under five world-wide, particularly in sub-Saharan Africa.
Two proteins, Pfs230 and Pfs48/45, are essential to the transmission process. These proteins form a complex on the surface of the parasite’s reproductive cells and are the targets of leading malaria vaccine candidates seeking to block transmission. Until now, the exact structure of this complex, including how the proteins fit together and how antibodies affect their function to prevent the spread of malaria, had not been known.
“This study provides the first molecular architecture of this complex as recognized by antibodies that can block malaria transmission, giving us the blueprint to design more precise vaccine candidates,” says Dr. Jean-Philippe Julien, Senior Scientist in the Molecular Medicine program at SickKids and co-corresponding author of the study.
A first look at the Pfs230:Pfs48/45 complex
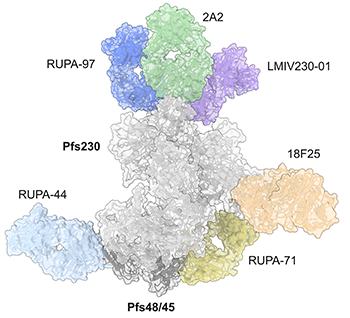
Current vaccine candidates target Pfs230 and Pfs48/45 to reduce malaria transmission. However, antibody responses observed during natural infection or in clinical trials with current candidate vaccines vary widely among individuals, underscoring the need for a deeper understanding of how these proteins function and how the immune system interacts with them.
Building on previous research by the global team into malaria and immune responses, scientists at Radboudumc first harvested the protein complex directly from the Plasmodium falciparum parasite using advanced genetic technology.
“These proteins were discovered back in the 1980s, but we still didn’t really know what they looked like,” says Ezra Bekkering, co-first author and PhD student at Radboudumc. “That’s because they’re difficult to produce.”
Radboudumc team members, supervised by study co-lead Dr. Matthijs Jore, cultured 30 billion malaria parasites over six months. To support the collaboration, Bekkering received a training grant to work at SickKids for several months to learn the biochemical and cryo-electron microscopy techniques that would be used in the study.
SickKids researchers, led by co-first authors and PhD students Randy Yoo and Sophia Hailemariam, then applied high-resolution cryo-electron microscopy to produce a 3D visualization of the complex while it was bound to six transmission-blocking antibodies. The imaging revealed how these antibodies interact with specific regions on the protein complex to prevent the parasite from spreading.
“Without a detailed understanding of how these antibodies interact with the complex, it’s hard to improve vaccine design,” says Julien. “Now, we and others in the field can use this information to develop next-generation vaccines that are more effective and more precise.”
Precision vaccines to combat malaria
At SickKids, Precision Child Health is a movement to deliver individualized care to every child. While vaccines are often seen as a “one-size-fits-all” approach, Julien argues that true precision means designing vaccines that are engineered to work for everyone.
“Our approach is about designing precision interventions that can be deployed at a global scale, maximizing transmission-blocking efficacy at a population level so we can help protect every child,” says Julien.
This precision approach to malaria intervention is part of a global effort to design a multi-pronged vaccination strategy for malaria that targets the essential components of all three aspects of the global burden: transmission, infection and disease severity.
“With the power of global collaboration, we can take on seemingly insurmountable health challenges,” says Julien.
This study was supported in major part by the Canadian Institutes of Health Research (CIHR), Radboudumc, Netherlands Organisation for Scientific Research, and the Federation of European Microbiological Societies.

