Researchers discover new way cells protect themselves from damage
Summary:
International effort reveals how cell compartments form a connected defense system to maintain cellular health.
An international team led by researchers at The Hospital for Sick Children (SickKids), Princess Margaret Cancer Centre, Dalhousie University, the University of Exeter (UK) and the Medical University of Vienna (Austria) has uncovered a surprising way compartments within cells work together to defend themselves against oxidative stress, a finding that could shift how we understand age-associated conditions such as diabetes and neurodegenerative diseases.
Published today in Science, the study reveals a newly identified mechanism between two key compartments of the cell (mitochondria and peroxisomes) that helps manage internal stress and damage, highlighting an overlooked contributor essential to maintaining cellular health.
A newly discovered protection mechanism
Mitochondria are often described as the powerhouses of the cell because they generate the energy needed for cells to function. This energy production also creates reactive oxygen species (ROS) as byproducts. These molecules can damage all parts of the cell, especially the mitochondria, and contribute to a condition called oxidative stress, an imbalance linked to diseases such as diabetes, neurodegeneration and cardiovascular conditions.
The international research team, led by Dr. Peter Kim at SickKids, discovered a new mechanism by which mitochondria protect themselves from the harmful effects of ROS. The study found that ROS generated by mitochondria can move to another compartment of the cell called peroxisomes through a newly identified contact point between the two organelles.
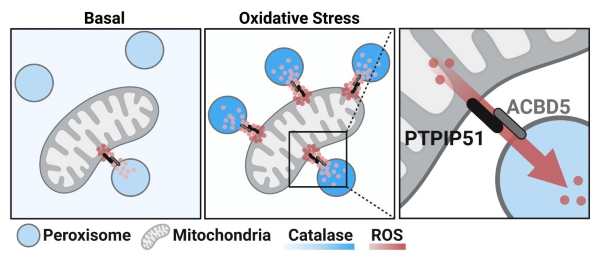
Through this mechanism, peroxisomes can spare mitochondria from oxidative stress by acting as specialized sinks for ROS produced in mitochondria. This challenges the long-standing idea that cellular defense is confined within individual compartments and is the first to show movement of ROS between organelles as a defense mechanism.
"Conventional dogma has always been that ROS are independently processed by organelles, but this work challenges that assumption by showing a mechanism by which peroxisomes directly mitigate mitochondrial ROS,” says Dr. Laura DiGiovanni, lead author and former graduate student in Kim’s Lab. “This discovery demonstrates that inter-organelle dynamics actively contribute to antioxidant defense, introducing a new perspective on how cells manage oxidative stress.”
A shift in understanding cellular defense and organelle cooperation
The team identified that the contact site is formed by two proteins: PTPIP51 on mitochondria and ACBD5 on peroxisomes. These proteins create a bridge that allows ROS to move directly between the organelles, sparing mitochondria while preventing damage to other parts of the cell. Both PTPIP51 and ACBD5 are disease-relevant proteins and have previously been linked to neurodegeneration.
While ROS play an important role in the body, too much can damage healthy cells, and too little can prevent the body from eliminating harmful ones, like cancer cells. By identifying the bridge that allows ROS to move between mitochondria and peroxisomes, the team has uncovered a spatially specific mechanism that helps maintain this delicate balance.
The discovery presents not only a potential new target for therapies but also challenges the assumption behind broad-spectrum antioxidant therapies. Targeting the precise cellular location where ROS are regulated, such as peroxisomes, may be key to developing more effective treatments.
“We know that dysregulation of mitochondrial ROS is linked to many diseases, so identifying these proteins that bring the two organelles together gives us a new entry point to explore how we might restore or strengthen this protective mechanism in cells,” says Kim, Senior Scientist in the Cell & Systems Biology program at SickKids and Professor in the Department of Biochemistry at the University of Toronto.
A multidisciplinary, international effort

This discovery was made possible by a global and multidisciplinary team of experts in cell biology, lipid biochemistry, proteomics and imaging. The project brought together collaborators from SickKids, University Health Network’s Princess Margaret Cancer Centre, Dalhousie University, and international partners from the University of Exeter and Medical University of Vienna.
“We are moving away from the idea that organelles operate in isolation and toward a more integrated view of how they communicate and coordinate to protect the cell,” says co-author Dr. Michael Schrader, Professor at the University of Exeter, and expert in organelle/peroxisome biology. “This is a fantastic example of how our independent discovery of ACBD5 and PTPIP51 as the bridging proteins has evolved into a multidisciplinary, international partnership, and we believe it has scope to change global understanding of how organelle interplay and cooperation impact on human health and disease.”
“This study is a powerful example of how collaboration across disciplines can uncover entirely new layers of biology,” says co-author Dr. Brian Raught, Senior Scientist at Princess Margaret Cancer Centre, whose expertise in using BioID to identify protein interactions solved the technical challenges for finding the contact site between mitochondria and peroxisomes.
This research is funded by Canadian Institutes of Health Research (CIHR), Natural Sciences and Engineering Research Council of Canada (NSERC), Austrian Science Fund, Biotechnology and Biological Sciences Research Council (UK), John Evans Leadership Fund grant from the Canada Foundation for Innovation, Ontario Innovation Trust, SickKids Restracomp Fellowship, and SickKids Foundation.

