Whole genome sequencing reveals new genetic marker for cardiomyopathy
Summary:
Using computational methods developed at SickKids, scientists identify tandem repeats are expanded in DNA of patients with cardiomyopathy.
In the first study to use whole genome sequencing to examine tandem repeat expansions in heart conditions, scientists at The Hospital for Sick Children (SickKids) have laid the groundwork for early detection of and future precision therapies for cardiomyopathy.

Cardiomyopathy is an inherited heart condition that impacts up to one in 500 individuals. The condition affects the structure and function of the heart and can ultimately lead to heart failure.
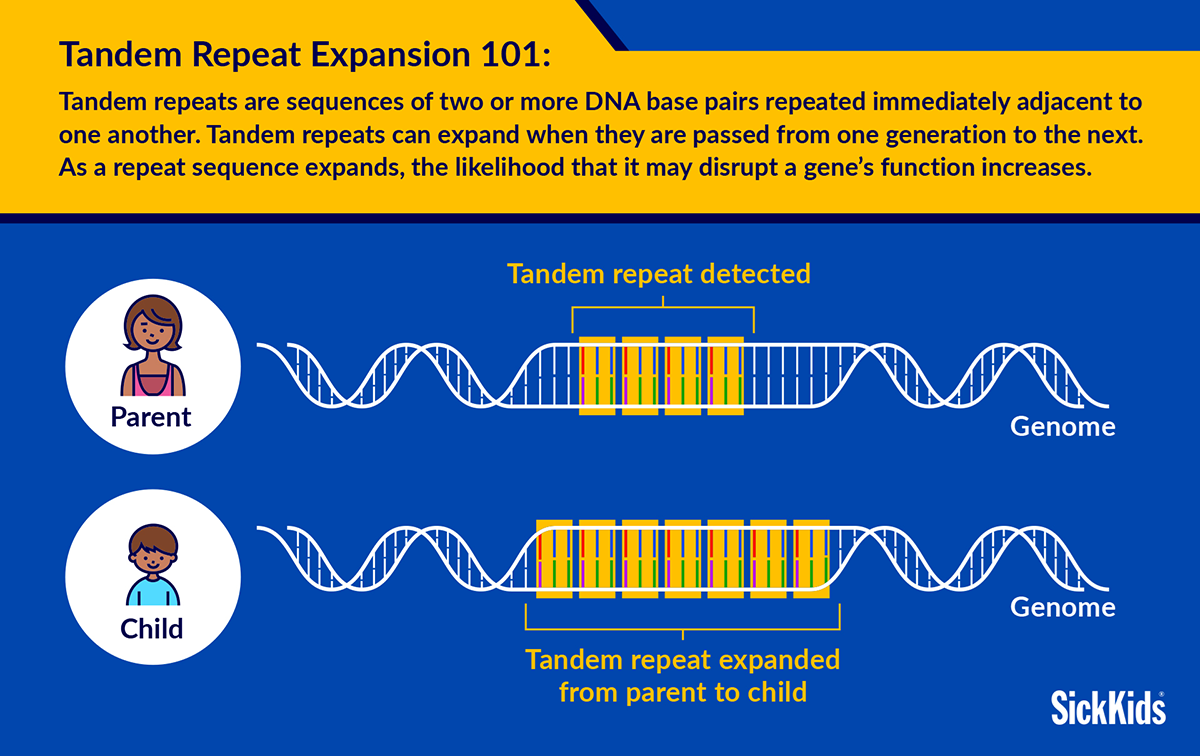
The SickKids-led study, published in eBioMedicine, part of The Lancet Discovery Science, indicates that tandem repeats – a form of genetic variation – are more often expanded in individuals with cardiomyopathy, and may cause four per cent of cases. Tandem repeat expansions (TREs) are known to contribute to over 60 conditions, often with a hereditary component.
“Previously the extent of TREs involvement in cardiomyopathy was unclear,” says study co-lead Dr. Ryan Yuen, Senior Scientist in the Genetics & Genome Biology program. “We now know another way to identify those who have a higher risk of developing the condition, which may eventually help clinicians diagnose patients, sooner.”
The research team also found a correlation between TREs in the gene DIP2B and a higher risk of developing cardiomyopathy. Uncovering this exact location of TREs could help in the development of precision therapies as part of Precision Child Health, a movement at SickKids to deliver individualized care for every patient.
“The better we understand the genetics of a condition, the more precise our treatments can become, informing care for people with cardiomyopathy and many other conditions with a genetic component,” says co-lead Dr. Seema Mital, Senior Scientist in the Genetics & Genome Biology program, Staff Cardiologist in the Division of Cardiology and Head of Cardiovascular Research at SickKids.
TREs provide new target for cardiomyopathy interventions
The team examined the genomes of 1,200 people with cardiomyopathy. When compared to study participants without a history of heart conditions, Yuen’s team found that tandem repeats that are rarely expanded in people are more present in individuals with cardiomyopathy. Researchers noted tandem repeats in the genes that primarily impact the heart – specifically in the left ventricle, which is responsible for pumping oxygenated blood into the body.
Researchers also noticed that children of parents with cardiomyopathy had larger TREs, a phenomenon known as “genetic anticipation.” When TREs become larger, there is a higher likelihood that a gene’s function will be disrupted. Larger TREs can also result in earlier onset and harsher symptoms, which is why scientists are investigating how to mitigate their growth.
“Our ultimate goal is to see if there is a way that we can stop or reverse these expansions in order to improve patient outcomes,” Yuen says. “By understanding the mechanism behind TREs, we could help reduce their genetic impact.”
Novel computational methods developed in Yuen’s lab and The Centre for Applied Genomics (TCAG) at SickKids enabled the team to fully examine the genome sequence data to locate exactly where and how large the TREs were in a person’s genome.
“With whole genome sequencing, we can look at TREs in the entire genome to help get a fuller picture of a person’s genetic predisposition,” says Dr. Aleksandra (Sasha) Mitina, a postdoctoral fellow in Yuen’s lab and first author of the study.
Using this technology, Yuen’s future research will apply similar methods to investigate regions of the genome that could help scientists develop treatments for conditions beyond cardiomyopathy.
This research was funded by the Government of Ontario, the Canadian Institutes of Health Research (CIHR), the Azrieli Foundation, the McLaughlin Centre of the University of Toronto, Ted Rogers Centre for Heart Research, the Data Sciences Institute at the University of Toronto, Heart and Stroke Foundation of Ontario, Bitove Foundation, Canada Foundation for Innovation, Genome Canada and SickKids Catalyst Scholar in Genetics.
The research team thanks all families participating in the Heart Centre Biobank registry who helped make this research possible.

