Developmental, Stem Cell & Cancer Biology
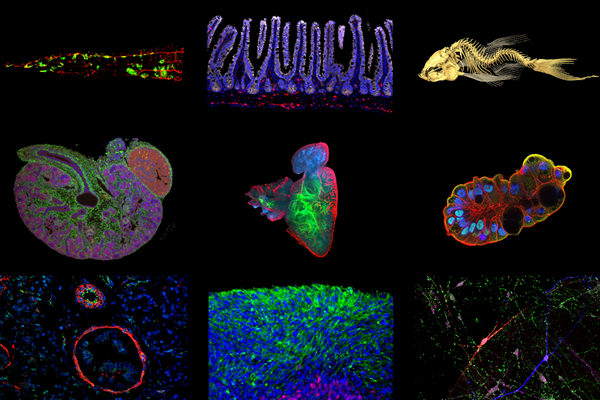
We're pushing the boundaries of stem cell biology. We're focused on understanding molecular genetic mechanisms affecting embryonic development, organ formation, tissue homeostasis and regeneration.
The Developmental, Stem Cell & Cancer Biology (DSCB) program is making an impact on paediatric health-care by translating our research into treatments for associated developmental disorders like childhood cancer, birth defects, and other paediatric diseases. We continue to develop new therapeutic approaches for children and youth through a better grasp of developmental genetics, genomics, and stem cell biology.
We are hiring! Applications open for Program Head, Developmental, Stem Cell & Cancer Biology (DSCB)
The Research Institute at The Hospital for Sick Children (SickKids) is seeking a dynamic research leader to be Head of the Developmental, Stem Cell & Cancer Biology (DSCB) Program, one of seven highly productive flagship research programs. The DSCB Program Head leads 20 independent investigators and more than 200 graduate students, postdoctoral fellows, administrative and research staff in both discovery-based and translational research activities that are focused on developing and studying innovative experimental models of human development and disease.
Our researchers use patient samples, stem cell and organoid platforms, as well as nematode, Drosophila, zebrafish, axolotl and mouse genetic models to interrogate pathogenic mechanisms underlying a diverse range of childhood disorders. Our common goal is to develop precision therapeutic strategies for paediatric cancer, neurodevelopmental and neurodegenerative disorders, congenital defects and rare childhood diseases.
The successful candidate will be appointed as a Senior Scientist in the Research Institute and is expected to qualify for an appointment at the Associate or Full Professor level (status only) in an appropriate department at the University of Toronto. The Program Head role is a five-year appointment, with the potential to renew this leadership role for a further five-year term following a successful Program review. At the end of their term as Program Head, the successful candidate will be eligible to maintain their Senior Scientist appointment.
Research team
Program head Dr. Brian Ciruna and the scientists that lead DSCB labs and core facilities are supported by over 225 graduate students, postdoctoral fellows, research associates, technicians and administrative staff.
You'll find our lab work regularly published in top peer-reviewed medical and scientific journals. Our scientists and trainees receive consistent funding from the Natural Sciences and Engineering Research Council of Canada (NSERC), Canadian Institutes of Health Research (CIHR), March of Dimes Foundation, Canada Research Chairs, NIH, Genome Canada, Canada Foundation for Innovation, and several other national and international organizations, illustrating the importance and impact of DSCB research.
We always welcome new trainees and staff that are eager to improve the health outcomes of children through cutting-edge biomedical research.
- Dr. Gabrielle Boulianne
- Dr. Chi-Chung Hui
- Dr. Janet Rossant
- Dr. Donna Wall
- Dr. Herman Yeger
- Dr. Ben Alman
- Dr. Rodrigo Fernandez-Gonzalez
- Dr. Binita Kamath
- Dr. Michael Taylor
Program Manager
Dr. Najeeb Siddiqui
Administrative Coordinators
- Janice Cheng
- Tirhas Okubazghi
- Nalma Uy
- Celeste Alora
- Michelle Turpin

May 6, 2025
When code meets CRISPR: Postdocs innovating in brain science
Two postdoctoral fellows working in the Li Lab are using different tools—computational modeling and gene-editing technology—to collaboratively unlock a better understanding of the developing brain.

March 4, 2025
SickKids staff awarded King Charles III Coronation Medal
Five SickKids staff were recognized for their contributions to Canadian health in the realms of nutrition, childhood cancer, kidney disease and lung development.
February 4, 2025
Research discovery halts childhood brain tumour before it forms
A SickKids research team has identified a critical event driving tumour growth in a type of medulloblastoma – and a way to block it.

Genetic models of human development and disease
We utilize diverse experimental organisms in their research, including C. elegans, D. melanogaster, zebrafish, planaria, and mice. By developing genetic models of numerous human diseases and cancer, we provide an unmatched platform for interrogating pathogenic mechanisms and performing genetic modifier and therapeutic drug screens.
Mechanisms of tissue patterning and organ development
Developmental signaling pathways – including BMP, Hh, FGF, Notch, TGFb, PCP, and Wnt, – play key roles in pattern formation and organogenesis. We examine the roles of these signaling pathways, as well as other regulatory factors in the development of diverse tissues and organs (e.g. blood, bone, brain, heart, gut, kidney, limb, lung, and skin).

Molecular regulation of cell fate decision, cell differentiation and cell death
We aim to answer a fundamental question in developmental biology - How do cells adopt their fate? Our team studies molecular switches that regulate a cell's fate, as well as mechanisms that program cells to die.
Stem cell biology, tissue engineering and regenerative medicine
We study the molecular genetic regulation of stem cell states, including embryonic, induced pluripotent, cancer, and multiple tissue stem cell models. Stem cells offer tremendous potential for repair and/or regeneration of damaged tissues and organs. We unlock how stem cells and organoids can be used in regenerative medicine and tissue engineering, such as spinal cord injury and bladder regeneration.

Browse a list of DSCB labs to learn more about the exciting research taking place in our program.

Centre for Advanced Single Cell Analysis
CASCA provides comprehensive training and expert consultation services to enable researchers to use the Fluidigm Helios Mass Cytometry (CyTOF), Fluidigm Hyperion Imaging Mass Cytometry (IMC) and BD Rhapsody single cell RNA sequencing (scRNA-Seq) platforms.
Flow Cytometry Facility
The FCF provides academic and corporate clients access to state-of-the-art analytical flow cytometers and high-speed cell sorting services. It offers comprehensive training and education as well as expert consultation services to enable users to enhance the scope and quality of their research using the FCF's technology.
Contact
Najeeb Siddiqui, Research Program Manager
686 Bay St, Toronto, ON M5G 0A4
416-813-6382













